Ⅰ. Introduction
Cancer is a prevalent disease in our society with 1,762,450 new cases projected in 2019(U. S. Breast Cancer Statistics, 2019). In women, about 30.00% of newly diagnosed cancers will be breast cancers (U. S. Breast Cancer Statistics, 2019; Wefel & Schagen, 2012). As of January 2019, there were 3.1 women Breast Cancer Survivors(BCS) in the United States and between 17~75% of them experience long-term cognitive deficits including deficits in memory, attention, processing speed and executive function(Von Ah, Jansen, & Allen, 2014). As cancer treatment becomes more efficient and effective, more BCS are living with cognitive impairment. These cognitive impairments result in decreased quality of life and functional abilities(Hines, Ramis, Pike, & Chang, 2014). Cognitive impairment in BCS is under diagnosed and leads to decreased participation in everyday activities that contribute to the individual’s wellbeing(Von Ah, Jansen, Allen, Schiavone, & Wulff, 2011).
Cognitive rehabilitation improves cognition, participation in everyday activities and quality of life(Ferguson et al., 2007; Willis et al., 2006). Occupational therapy practitioners are well prepared to provide cognitive rehabilitation for cancer survivors (Bail & Meneses, 2016; Baxter, Newman, Longpré, & Polo, 2017; Pergolotti et al., 2015; Player, Mackenzie, Willis, & Loh, 2014). They may remediate cognitive deficits by having clients practice cognitive skills needed to improve attention, processing speed, memory, and executive function. One means of providing patient- centered or individualized cognitive rehabilitation for BCS is by practicing cognitive skills with computer- assisted training(Von Ah et al., 2012).
Computer-assisted cognitive training has been delivered to improve cognition for populations such as middle and older adults, stroke patients, patients with mild dementia, cancer, and BCS(Bray et al., 2016; Chen, Mao, Li, Zhao, & Zhang, 2015; Kesler et al., 2013; Lee, Jang, Bak, & Yoon, 2013; Li & Li, 2014; Van Vleet, DeGutis, Dabit, & Chiu, 2014; Von Ah et al., 2012; Wolinsky, Vander Weg, Howren, Jones, & Dodson, 2013; Yoo, Yong, Chung, & Yang, 2015). A variety of computer software has been used such as Dynamic Brain, Insight from Posit Science, Luminosity, and RehabCom(Bray et al., 2016; Chen et al., 2015; Kesler et al., 2013; Klingberg, 2010; Lee et al., 2013; Yoo et al., 2015). Previous computer-assisted cognitive training programs for BCS focused on improving a variety of cognitive skills such as processing speed, executive function including working memory, cognitive flexibility, multitasking, planning, and attention, and visual precision, divided attention, working memory, field of view and visual processing(Bray et al., 2016; Kesler et al., 2013; Von Ah et al., 2012). These programs ranged widely in length from 6~15 weeks and used visual tasks(Bray et al., 2016; Kesler et al., 2013; Von Ah et al., 2012).
Computer-assisted cognitive training can be delivered concurrent to usual and customary cognitive rehabilitation. It can be beneficial by amplifying or speeding results from usual and customary treatment thereby leading to decreased number of treatment visits required and/or a quicker recovery for BCS. In addition, researchers have concluded that this type of rehabilitation for cognitive deficits is feasible as it is an entertaining home based program that requires no travel and the difficulty of tasks increases as the participant’s skill level improves(Kesler et al., 2013).
The state of computer software today is such that programming exists for patient-centered cognitive training that addresses skills adversely affected by cancer treatment and audio exercises are available. Kesler et al.(2013), noted that a limitation of their study was it did not include auditory exercises due to a lack of auditory computer exercises at the time. They stated that cognitive training programs that include both visual and auditory exercises might be more comprehensive and result in larger effects. Also at issue is whether training on computer exercises transfers to real-world tasks(Klingberg, 2010).
It is only in recent years that computer-assisted cognitive training could be utilized. More recent is the ability to deliver audio exercises through computer programming. There is a gap in the literature as to the effects of auditory exercises in computerassisted cognitive training. The present study addresses this gap. At this end, the aims of this study were: (1) to compare the effects of a computerassisted cognitive training with visual exercises(visual group) to one with audio exercises(audio+visual group) on working memory, perceived cognitive deficits, quality of life(QOL) and engagement in meaningful activities for breast cancer survivors; and (2) to explore the relationship between engagement in meaningful activities, cognitive deficits and QOL for breast cancer survivors.
Ⅱ. Methods
1. Research Design
A prospective randomized single blind design was used in this pilot study. Participants were randomly assigned to two different intervention groups. One group received computer-assisted visual exercises from HAPPYneuronPro and the other group received audio and visual exercises (HAPPYneuronPro, n.d.). Four outcomes were measured before and after one-month of treatment.
2. Participants
Participants were recruited from five different breast cancer support groups. Each researcher visited two support groups to present the proposed study and collect contact information of potential participants. A written description of the proposed study was sent to the director of the fifth support group and potential participants contacted the first researcher. When potential participants were contacted, they were given further information about the study. If they wanted to participate, a time and place for pretesting was arranged. Inclusion criteria for participants were they must be a breast cancer survivor and report cognitive problems which they attributed to their breast cancer treatment. Exclusion criteria were persons who cannot read or understand spoken English or who have disorders that may affect their cognition including major mental disorder, central nervous system disorders, Alzheimer’s disease, dementia, developmental delay, traumatic brain injury or cerebral accident. According to a priori power analysis set at power of 0.8, alpha level of 0.05, and moderate-high effect size of 0.3, a total of 24 participants were recruited in this pilot study.
3. Measures
Four measures were administered pre and post computer-assisted training. Measures included an objective cognitive function for working memory, a subjective cognitive function of perceived cognition, a quality of life measure for cancer survivors, and a measure of participation in meaningful activities. Working memory was measured by the digit span, shown to discriminate between BCS and controls(de Paula, Mallory-Diniz, & Romano-Silva, 2016). It takes approximately 10 minutes to administer and has high reliability(.891) for forward span and low for backwards span(.598)(de Paula et al., 2016). Subjective cognitive function was measured with the 37 item Functional Assessment of Cancer Therapy- Cognitive Function(Wagner, Lai, Cella, Sweet, & Forrestal, 2004). The FACT-Cog contains 4 subscales of perceived cognitive impairment, comments from others, perceived cognitive abilities, and impact of cognition on quality of life which are summed to provide a score. The Fact-Cog demonstrates acceptable test-retest reliability(.707) and validity (.762)(Wagner et al., 2004). Quality of life as it relates to cancer survival, was measured with the 41 item Quality of Life Instrument(CANCER PATIENT/ CANCER SURVIVOR VERSION)(Ferrell, Hassey-Dow, & Grant, 1995). The overall QOL-CS tool test re-test reliability is .89 and the overall QOL-CS correlation with the FACT-Cog is .78(Ferrell, Dow, & Grant, 1995). Engagement in meaningful activities was assessed with the Engagement in Meaningful Activities Survey (EMAS) to calculate how often a subject participates in 12 activities. Activities include self-care, reflect the kind of person he/she is, creativity, sense of accomplishment, contribute to feeling competent, valued by other people, to help other people, pleasurable, give a feeling of control, express values, give satisfaction, and provide a challenge. It has been shown to have moderate test-retest reliability(r = .560) and good internal consistency(Cronbach’s alpha = .890)(Eakman, Carlson, & Clark, 2010) and has the potential to validly measure subjective qualities of meaningful activity participation(Eakman, 2012).
4. Research Protocol
This study and consent form were approved through the first researcher’s affiliated university institutional review board. Prior to any data collection participants provided written informed consent at one of three sites based on participant convenience. Participants were administered the instruments described above which took approximately 45 minutes. Participants were then instructed on how to access and perform the computer-assisted exercises. An email reminder was sent to the participants the night before their exercises started and as needed for them to complete computer exercises for 30 minutes a day, five out of seven days a week. Each exercise session consisted of 10 different exercises run for up to 3 minutes each. The level of difficulty for exercises was individualized; once a participant achieved a 100.00% percent score at a specific level, the next level of exercise was presented.
Participants were randomly assigned to either the visual or audio+visual group. The software features nine different categories of cognitive exercises. Three of the exercise categories use auditory input and are named auditory, verbal and visual memory, and verbal memory. The other six categories are executive functioning, processing speed, spatial memory, visual attention, visual memory, and visual and spatial abilities. The visual group was assigned computer exercises only from the six categories that did not employ auditory input. The audio+visual group was assigned computer exercises from all nine categories. Both groups did 30 minutes of assigned computer exercises but due to the fact that the audio+visual group had exercises from all nine categories every week, they performed fewer exercises from the six non auditory categories.
At the end of the one month of exercises, all tests were repeated. Participants received a $25 Walmart gift card for both the pre and post-test data collection meetings as well as for each of the 4 weeks of computer exercises. Total participant reimbursement was $150 in Walmart gift cards and was dispersed on the posttest data collection meeting to all participants who completed the program.
5. Data Analysis
Once data were collected, a data screening process was conducted to ensure the validity of the data, conduct recoding, assess missing-ness, and to test assumptions of the data. There were no issues regarding the validity of the data. There were no missing data values in the overall dataset. To address Aim 1 for this study, each of the four pretest and posttest outcome measures were assessed by visual and audio+visual group participants using a 2 x 2 mixed analysis of variance(ANOVA). Interaction effects were assessed to determine if group means over time on the outcome are significantly different. Pairwise comparisons of groups and time were assessed using a Bonferroni correction to account for multiple tests. The effect sizes for this type of analysis is partial eta squared(η2p). Marginal Homogeneity test was conducted to examine whether there was any difference on the categorical frequency of activities from pretest to posttest in each group. Crosstabulation using Fisher’s exact tests was conducted to assess activity frequency differences between the two groups at each time point, and the effect size for this test was expressed as Cramer’s V. In order to address Aim 2 of this study, the correlation for pretest, posttest, and changes of measures in perceived cognitive function, QOL, and engagement in meaningful activities were examined using Spearman’s rho non-parametric correlation to account for the small sample size. The effect sizes for this type of analysis produce a rho (ρ) which is analogous to an r effect size correlation in terms of interpretation. The data was managed and analyzed using IBM SPSS v25. The alpha level was set at .05.
Ⅲ. Results
1. Participants
A total of 24 participants(13 in the visual group and 11 in the audio+visual group) were included in the study. Half of the participants were Caucasian (N = 12). The rest of the participants were African American(N = 9) and Hispanic (N = 3). Participants ages ranged from 28 to 71 years with average age of 52.04 years(SD = 10.61). Years since last treatment ranged from 0.5 to 15.0 with average years of 5.51(SD = 4.77).
2. Primary Analysis
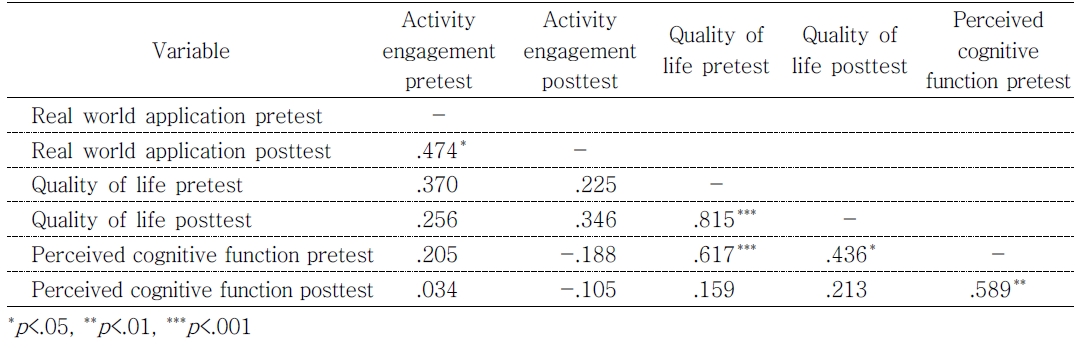
Aim 1 compared differences on the outcomes between the two groups from pretest to posttest. Perceived cognitive function, in Figure 1, participants in the visual group reported significantly better cognitive function in the posttest test(M = 91.62, SD = 21.75) than that in the pretest(M = 74.48, SD = 29.00), p = .010. There was an improvement trend in the audio+visual group between pretest(M = 80.18, SD = 19.22) and posttest(M = 93.73, SD = 21.97), but the result was not significant(p = .054). With respect to QOL shown in Figure 2, participants in the visual group reported significantly higher score in the posttest test(M = 6.42, SD = 1.27) than that in the pretest(M = 5.60, SD = 1.76), p = .004. However, the audio+visual group did not appear to impact QOL from pretest(M = 6.44, SD = 1.27) to posttest(M = 6.69, SD = 1.23). In addition, no significant differences were found between two treatment groups at either time point.
Figure 1
Quality of Life Between Visual and Audio+Visual Groups in Pretest and Posttest.
Note. All values are mean±SD(n=13 in the visual group and n=11 in the audio+visual group). Group*time interaction: F(1, 22) = 2.33, p = .141, η2p = .096. Time effect: F(1, 22) = 8.01, p = .01, η2p = .267. *, significantly different from pretest in the visual group, p < .05.
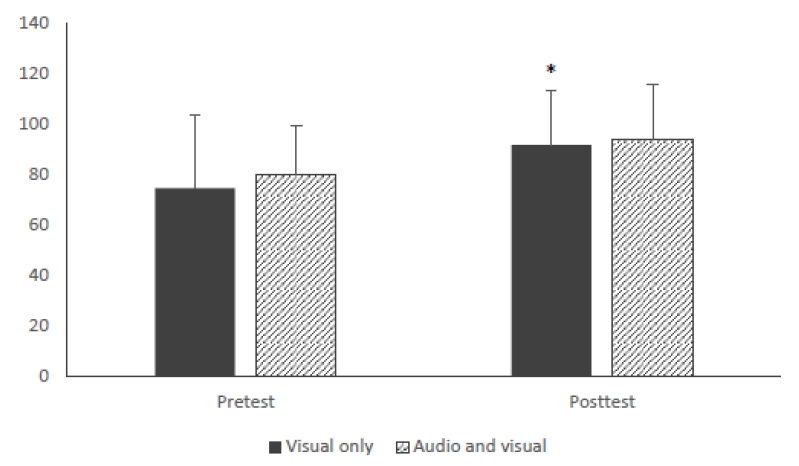
Figure 2
Perceived Cognitive Function Between Visual and Audio+Visual Groups in Pretest and Posttest.
Note. All values are mean ± SD(n= 13 in the visual group and n = 11 in the audio+visual group). Perceived cognitive function group*time interaction: F(1, 22) = .16, p = .695, η2p = .007. Time effect: F(1, 22) = 11.56, p = .003, η2p = .344.*, significantly different from pretest in the visual group, p < .05.

For the other three outcomes, shown in Table 1, the interactive effect for the total working memory was not significant, F(1,22) = .01, p = .922, η2p = .000. Similarly, the interactive effect for the working memory length was not significant, F(1,22) = .52, p = .477, η2p = .023. When it comes to engagement in meaningful activities, the interactive effect was not significant as well, F(1,22) = .52, p = .480, η2p = .023. Furthermore, nether time effect nor group effect showed significant findings on the three outcome measures. In the EMAS pretest, however, participants in the audio+visual group(M = 36.36, SD = 5.35) reported significantly higher score than those who were in the visual group(M = 31.08, SD = 6.20), p = .038. Taken together, the above results suggest that visual intervention improved participants’ perceived cognitive function and quality of life, whereas no significant improvement was observed in the audio+visual group. In the posttest, the three outcomes in the visual intervention were not significantly different from those in the audio+ visual group.
With regard to the engagement in meaningful activity variable, most participants reported moderate frequency of activities in both pretest(N = 17, 70.80%) and posttest(N = 15, 62.50%). Engagement scores in meaningful activities were then classified into low(< 29), moderate(29~41), and high(> 41) frequencies of activities. The results, seen in Table 1, indicate significantly higher frequency of activities in the audio+visual group than that in the visual group for the pretest, Fisher’s exact test = 6.23, p = .031, Cramer’s V = .538. Specifically, none of the participants in the visual group reported high frequency of activities, whereas none of the participants in the audio+visual group reported low frequency of activities in the pretest. No significant difference was found in the posttest between the two groups, p = .725. However, it is worth noting that two participants had high frequency of activity from low or moderate activity engagement after undergoing the visual intervention, whereas one participant in the audio+visual group reduced activity frequency from moderate engagement to low engagement.
Aim 2 assessed the relationships between engagement in meaningful activities, cognitive deficits and QOL. As shown in Table 2, perceived cognitive function in the pretest was significantly associated with QOL life in both pretest(ρ = .617, p < .001) and posttest(ρ = .436, p < .05). Participants who reported greater perceived cognitive function in the pretest also reported better QOL in both pre and posttests. However, the relationship between these two variables was not observed in the posttest(ρ = .213, p > .05). They were also not related to engagement in meaningful activities at either time point, all p > .05. Additionally, the change scores were calculated as differences from pretest to posttest measures, and the relationships among the changes were examined using Spearman’s rho correlation. None of the outcome changes were associated with each other.
Ⅳ. Discussion
This pilot study addressed two aims. The first aim was to determine effects of a visual computerassisted cognitive training program compared to an audio+visual program on working memory, perceived cognition, QOL and engagement in meaningful activities. We saw no improvement in participants’ working memory, as measured by the digit span, in either program. Previously, working memory has been considered static and improvements accomplished through learning memory techniques. More recent research indicates that implicit working memory training results in neuronal changes (Klingberg, 2010). Although, working memory was addressed with computer-assisted cognitive exercises in our study, exercises were not exclusive to working memory and only up to 3 minutes per exercise were programmed per day. Despite reporting several of their exercises being targeted to improving working memory, Kesler et al.(2013), also found no improvement in working memory. The visual program resulted in significantly improved post-test scores for perceived cognition and QOL. Other researchers using computerassisted cognitive training have shown similar improvements in perceived cognitive using the FACT-Cog and QOL measured with the QOL-CS (Bray et al., 2016; Von Ah et al., 2012). The second aim was to examine the relationship between engagement in meaningful activities, cognitive deficits and QOL. The only significant association determined was that of perceived cognitive function in the pretest to QOL in both pretest and posttest.
Our study demonstrated higher-level engagement in meaningful activities by the audio+visual group at pretest, no significant difference between the two groups at post-test, and no significant change scores for either group. Participants worked a minimum on the computer exercises of 2 1/2 hours a week and intervention was for only one month. They may not have found performing computer exercises meaningful or the amount of time spent on the exercises may have limited their time to do activities they find more meaningful. Researchers using computer-assisted cognitive training for 12 weeks concluded that a longer duration of intervention time is needed to assist in transference of cognitive improvement to real-world behaviors (Kesler et al., 2013).
Researchers also sought to understand the relationship of engagement in meaningful activities, cognitive deficits and QOL. Although a significant association existed between perceived cognitive function in the pretest to QOL in both pretest and posttest, no association was found between engagement in meaningful activity with perceived cognition or QOL. In contrast, Von Ah and Tallman (2015) found no correlation between QOL and perceived cognition but did find significant relationships between QOL scores and depressive symptoms, fatigue, anxiety, and sleep. They used an overall QOL measure whereas the measure used in this study was a QOL measure normed for BCS.
There are a number of study limitations. We had a small number of participants and findings cannot be generalized to persons outside the study. Technological issues may have affected some participants’ participation and or performance. At least two used their cellphones to perform their exercises because they did not have internet access. Even though a tablet was made available to them, they lived many miles from a free Wi-Fi location and decided they could not incorporate such a lengthy journey into their schedule. Participants who used their cellphones seemed to have more difficulty interfacing with the computer exercises and the smaller size of their screen may have adversely affected their performance. Several participants reported difficulty with one of the audio exercises freezing up on them. Last, some participants required more reminders to complete their exercises in a timely fashion and the researcher did remind them.
Future research should explore incorporating audio input through different means to improve cognition for BCS as well as the benefits of the use of other sensory input. Further, computer-assisted training is only a component of cognitive rehabilitation; what constitutes best practice for BCS needs further exploration with larger studies. Functional assessments to measure performance of instrumental activities of daily living requiring higher level cognitive ability should be employed pre and post-rehabilitation to aid in our understanding of the complex relationship of functional performance, cognitive deficits and quality of life for BCS.
Ⅴ. Conclusion
Computer-assisted cognitive training using visual exercises appears to improve perceived cognitive function and quality of life for BCS, whereas visual plus audio training program does not appear to improve perceived cognitive function. There were also no differences between the two training programs in the posttest. Moreover, there exists a positive relationship between perceived cognitive function and quality of life for BCS. Computer-assisted cognitive training specific to the cognitive skills adversely affected for BCS offers a method occupational therapists can use for home programs.