Ⅰ. Introduction
Dysphagia is defined as difficulty swallowing food, liquid, and/or saliva. Thin liquid dysphagia is the most common form (Feinberg, Knebl, Tully, & Segall, 1990). According to results from the 2012 National Health Interview Survey, dysphagia annually affects one in 25 adults in the United States (Bhattacharyya, 2014). Dysphagia can result from a variety of etiologies, for example stroke and brain injury, which are common diagnostic groups seen in Inpatient Rehabilitation Facilities (IRFs). On average, dysphagia affects over 40% of patients in the first two weeks after a stroke (Paciaroni et al., 2004). Additionally, individuals who have experienced a severe Traumatic Brain Injury (TBI) report between 61-90% prevalence of dysphagia (Mackay, Morgan, & Bernstein, 1999; Terre & Mearin, 2007).
Dysphagia has been associated with age, specifically resulting in negative implications for older people including adverse impacts on recovery (Miller & Patterson, 2014; Roy, Stemple, Merrill, & Thomas, 2007). A common issue associated with dysphagia is aspiration, or when food or liquid passes through the vocal folds into the airway (Hammond & Goldstein, 2006). Individuals who aspirate are at a higher risk for developing pneumonia. Aspiration pneumonia requires immediate medical intervention as it can cause serious illness and is expensive to address (Marik, 2001; Marik & Kaplan, 2003). Once thin liquid aspiration is detected, thickened liquids are commonly recommended as a compensatory strategy to prevent aspiration. Patients have reported to dislike the thickened liquids and clinicians have observed non-compliance of nearly 50% (Garcia, Chambers, & Molander, 2005; Shim, Oh, & Han, 2013). Subsequently, dehydration can develop (Gillman, Winkler, & Taylor, 2017). An alternative option to thicken liquids is not to ingest anything by mouth. Prohibiting food or liquid by mouth, can then result in measures such as invasive medical procedures such as nasogastric, percutaneous gastrointestinal, or intravenous tubes to be placed. While these would then be necessary for nutritional needs, all carry specific risks and expenses (Kurien, McAlindon, Westaby, & Sanders, 2010).
Considering these circumstances, Panther and colleagues at the Frazier Rehabilitation Hospital developed a “Free Water Protocol” (FWP; Panther, 2005). This treatment program allowed patients with thin liquid dysphagia the option to drink water under the supervision of healthcare professionals, usually between mealtimes. There are two main reasons why providing water to those with aspiration risk is appropriate. First, the recommendation is very small amounts of water and second because of the neutral pH value of water. Liquids with a pH less than 2.5 are recognized to cause pneumonitis. However because water is of a neutral pH, if aspirated, it osmotically passes out of the alveoli into the bloodstream (Caruso, Denari, Ruiz, Demarzo, & Deheinzelin, 2009; Coyle, 2011; Marik, 2001). If water alone is safe for patients to drink who might aspirate, then protocols allowing for water might reduce urinary tract infections, increase hydration and improve quality of life of patients, for those on modified diets (Gillman, Winkler, & Taylor, 2017; Murray, Doeltgen, Miller, & Scholten, 2016).
The free water protocol’s provision of care includes a short set of rules that was developed for an IRF setting (Panther, 2005). These guidelines are consistent with the intensity and goals of the IRF where patients typically receive a 30-60 -minute speech therapy session, daily, usually during the mealtime hours. The free water protocol suggests oral intake of water 30 minutes after a meal and after oral care. Thus, the Speech and Language Pathologists (SLPs) could easily offer water to clients when they finish the therapy session.
There are research studies that support use of FWP protocols. However, the research includes relatively small samples, limited diagnostic groups, and selective settings (Carlaw et al., 2012; Frey & Ramsberger, 2011; Garon, Engle, & Ormiston, 1997; Karagiannis & Karagiannis, 2014; Murray et al., 2016; Panther, 2005; Scott & Benjamin, 2010). Additionally, there is limited information on rehabilitation-specific outcomes for those in a FWP, and especially for the geriatric population and with mixed results (Gillman et al., 2017). Therefore, it is important to understand more about the geriatric population in an IRF, participating in a FWP and collect information on rehabilitation and quality care outcomes.
The purpose of this retrospective study was to gain information on the implementation and expansion (e.g., feasibility) of a FWP at an IRF and report descriptive information on outcomes in this cohort, such as: age, medical diagnoses, Acute Care Hospital Transfer (ACHT) rates, discharge destinations, and length of time on the FWP. Our hypotheses include: (1) the FWP would be feasible and (2) that the outcomes collected would report diverse diagnostic groups enrolled in the FWP without events such as ACHTs.
Ⅱ. Methods
1. Procedures
The FWP clinical program that was developed at Kessler Institute for Rehabilitation was initiated in 2008 by the SLP clinical management team as a quality improvement initiative, to improve the quality management of dysphagia care. The program is based on the work of the original 1984 protocol (Garon et al., 1997; Panther, 2005) and involved distributing water after meals, after oral care, or during the therapy sessions as recommended by the therapist. The therapists would be required to document these clinical sessions in the patients’ respective daily notes. Because the documentation occurred, when the program team and management were interested in understanding more about the patients being enrolled into the FWP and collect outcomes, it was doable. The Institutional Review Board at Kessler Foundation approved this retrospective study as an expedited review (IRB# E-881-15) because de-identified information was used.
2. Participants
Clinical referrals included patients with a diagnosis of thin-liquid dysphagia, confirmed with a non-instrumental clinical dysphagia evaluation. For most, this was followed by a standardized evaluation, called a Video Fluoroscopic Swallow Study (VFSS), which also objectively assess the severity of the verify dysphagia. A certified licensed speech language pathologist completed both assessments. Care protocols for dysphagia assessment used have been previously reported (Heckert, Komaroff, Adler, & Barrett, 2009). In addition, SLPs would also get referrals to the FWP for patients with: (1) a recent aspiration pneumonia, (2) respiratory compromise, (3) terminal disease such as metastatic cancer or degenerative disorders, or (4) other severe and medically complex swallowing conditions. Consultation with the patient’s medical doctor was completed for these referrals, prior to starting on the FWP.
Patients who were ventilator-dependent, comatose (vegetative or in minimally conscious states), had current or active pneumonia (chart review as well as chest x-ray results were used); or those who had an absent swallow reflex were excluded. Participants also had to be medically stable and no recent history of pneumonia. Participants could not have excessive coughing in reaction to aspiration. In addition, usually participants could not have presence of lingual or oral thrush.
3. Data Collection
Demographic and clinical data were retrieved from an electronic medical record called Healthcare Management Systems (HMS) and recorded in Excel. This included each participant’s age, medical diagnosis, discharge destination, and length of time on the FWP. Clarification of whether an ACHT happened required use of an additional dataset. This information was retrieved from a national database called eRehab (erehabdata.com), maintained to track quality standards in post-acute rehabilitation.
4. Data Analysis
Descriptive statistics were completed to determine the following: mean age of the participants, medical diagnoses (tallied, then reported in percentages by group), ACHT rates (tallied, then reported in percentages for study participants and institution), disposition after IRF discharge for each person (tallied and reported in percentage by type of facility), length of time in the FWP (mean, standard deviation) reported in days. We determined feasibility as measured by implementation and expansion (Bowen et al., 2009).
Ⅲ. Results
Implementation and expansion are two general areas of focus addressed by feasibilities studies. Implementation concerns the likelihood and manner to which the program was able to be used as it was planned and as proposed by the original group. The program in this study was based on the Frazier protocol, which was delivered as intended; free water was provided by the therapist after meals and after oral care to the participants meeting inclusion. This program started small with one therapist. After training staff, the inpatient speech department successful expanded it to all floors of the hospital. They clinically administered the protocol for over 6 years and with over 500 people of different medical conditions.
This cohort was mostly an older adult population (M = 68.43, SD = 15.11). Table 1 displays 575 participants in the FWP by diagnoses. There was a variety of diagnostic groups represented including brain injury (BI), cardiac, stroke (ischemic and hemorrhagic, including subarachnoid hemorrhage), spinal cord injury (SCI), orthopedic injuries, critical illness, general debility, multiple sclerosis (MS), Parkinson's disease (PD), and Guillain-Barré Syndrome (GBS). There were eleven people with ICD-9 codes for intracerebral hemorrhage that were non-traumatic in-nature and therefore were grouped into the stroke diagnosis category.
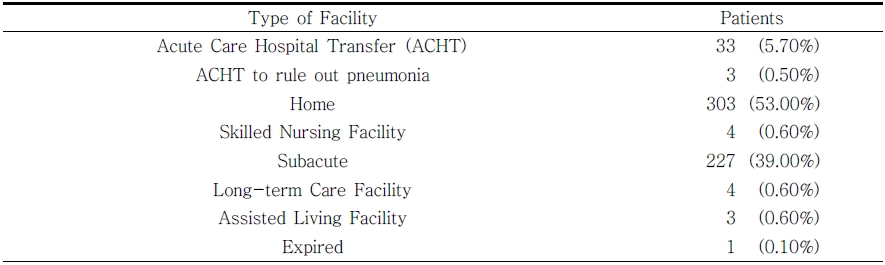
Table 2 reports six percent (33 of 575) of the participants in the FWP required a “transfer” or returned to the acute care hospital during their IRF stay; 3 of which were to rule-out pneumonia. Although a matched sample would be needed for a formal statistical comparison, we completed exploratory look at hospital internal data reports. During the years of this retrospective review, the 6% transfer rate is lower than an average base rate of 14% (Table 3).
One person expired during the IRF stay, however the cause of death was ruled to be unrelated to dysphagia. Regarding discharge, 303/575 or 53% of participants in the FWP were discharged to home. This is lower than the average base rate of 69.4% at the hospital during the years of recorded review, although again, this comparison was not with a matched sample.
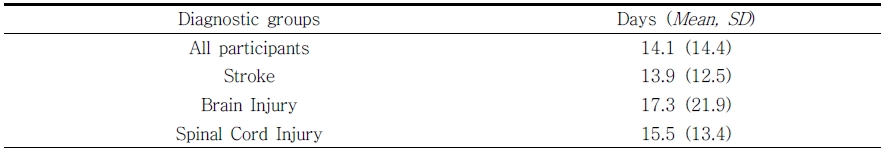
The mean length of time a participant enrolled in the FWP was 14.1 (SD = 14.4) days as seen in Table 4 (range 1 - 132 days). Most of the participants (83.5%) were enrolled between 1-20 days. We looked more closely at the four outliers with the longest length of time in the FWP. These participants were enrolled between 111-132 days. Each of those four participants’ diagnoses were reviewed and this visual analysis revealed that two of the four were classified as BI and the other two as stroke survivors. All four people were all living with multiple comorbidities.
Ⅳ. Discussion
Feasibility studies are necessary for researchers to know if a protocol needs modification, for a specific population or practice setting. Addressing questions of feasibility and quantifying it appropriately is an essential step to complete prior to efficacy testing and for quality management. In this retrospective report, we discovered that a FWP was able to be implemented and scaled for use throughout the entire IRF (expansion). As the results concluded, the protocol was provided for 6 years and with over 500 patients with multiple diagnoses. This cohort was an older group of people, mostly stroke survivors. They tolerated or participated in the FWP, on average for 14.1 days. Usually a stroke survivors’ length of stay in an IRF is 14 days. Thus, implementation of this protocol in an IRF appears to be an appropriate setting and for the appropriate patient population.
We also found the ACHT rates were lower than our inpatient facility average during this period. Fifty three percent of the FWP participants when home and for those who required a transfer back to acute care, it was a 6% rate. This seems to be a low rate considering the complexity of patients who typically have dysphagia. For example, patients with enteral tube dependency and dysphagia are less likely to be discharged to home and have significantly higher associated medical expenses (Bonilha et al., 2014; Roberts et al., 2014). These variables are important to hospital administration and team members because of costs. For example, transferring patients back to the acute care setting is expensive, and disrupts their rehabilitation plan, continuity of care, and progress (Carlaw et al., 2012; Gillman et al., 2017).
There are limitations to this study. Since this is a clinical dataset, not intended initially for research, data on race or other variables typically seen in a research study were not collected. Also, this report solely provides outcomes of a descriptive clinical initiative and therefore cannot make final predicative conclusions. Despite, results of this research contributes to the growing body of evidence that supports the implementation of FWPs, carried out at an IRF (Gillman et al., 2017).
Future work should be completed using experimental studies and on topics related to whether low incidence of FWP complications have direct impact on ACHT and/or discharge destination. Additionally, it might be necessary to include individuals in experimental studies with comorbid complexity (high case-mix index scores) because it is common to live with more than one health condition. It may be appropriate for studies to also investigate costs and the effect of thin liquids on swallowing practice in dysphagia recovery.
Ⅴ. Conclusion
This retrospective study concludes successful feasibility of a FWP in a rehabilitation facility and reports descriptive outcomes such as discharge destination. This study adds to the literature already existing on the FWP. The impact of the FWP on readmissions and other outcomes requires a future experimental, prospective study, in order to make those conclusions.