Ⅰ. Introduction
A child’s stagnating or declining function in Activities of Daily Living (ADL) is one of the first clinical indicators of progression of rare disorders. Congenital muscular dystrophy and Barth syndrome are both rare disorders among children (Lim, Bendixen, & Mann, 2016). Congenital muscular dystrophy is a genetic disorder and characterized by lack of the protein dystrophin in muscles (Kirschner, 2013). Congenital muscular dystrophy is present at birth and is marked by hypotonia, generalized muscle weakness, and frequently, multiple contractures (Shah, 1998). In addition, intellectual impairment may be present (Sparks & Escolar, 2011). Barth syndrome is a genetic disorder linked to the X chromosome and found only in boys (Clarke et al., 2013). Barth syndrome has a similar symptom of muscle weakness like congenital muscular dystrophy. Barth syndrome is characterized by slow growth, cardioskeletal myopathy, and chronic or cyclic neutropenia (Mazzocco, Henry, & Kelly, 2007). As muscle weakness in children with theses rare disorders progresses, increased caregiving is required to accommodate decreased levels of independence in all areas of ADL (Thomas, Guerrero, Hines, Ashley, & Brada, 1995). Therefore, choosing a relevant ADL assessment is crucial to provide effective occupational therapy services for children with rare disorders. A relevant and evidence-based assessment allows occupational therapists to focus their interventions and caregiver training on the most important ADL while measuring patient progress.
The Modified Barthel Index (MBI) is a commonly used assessment to measure the level of ADL independence (Shah, 1998). The MBI assesses functional performance by measuring the level of independence one has in performing ADLs (Schiavi et al., 2018). The convenience of the MBI is evident as occupational therapists often use this instrument for evidence-based practice across diagnoses, settings, and age ranges (Choe, Kim, Kim, & Im, 2018; Ohura et al., 2017). Occupational therapists use the MBI to determine the occupational needs of their client and to track progress in occupational therapy service (Schiavi et al., 2018). The MBI has been successfully used as an assessment tool, outcome measure, and to support clinical decision-making by occupational therapists when working with multiple populations with chronic diseases, such as neurological disorders (Fricke & Unsworth, 1997; Ohura et al., 2017; Schiavi et al., 2018). Research indicates that the MBI is highly valid and reliable for use in individuals of all ages with spinal cord injuries and demonstrated good internal consistency in patients with stroke (Anderson et al., 2008; Hobart & Thompson, 2001; Küçükdeveci et al., 2000).
As a valid, reliable, evidence-based, and convenient tool, the MBI has been supported by clinical research. One study evaluated the psychometric properties of a Shah version of the MBI in the geriatric population (Tagharrobi, Sharifi, & Sooky, 2011). The internal consistency conducted in this study was found to be .96-.99 (Tagharrobi et al., 2011). The known-groups approach revealed its validity, where the p-value is less than .0001 (Tagharrobi et al., 2011). Overall, the study concluded the MBI is also a reliable and valid assessment in the geriatric population (Tagharrobi et al., 2011). The Korean version of the MBI was used in a study with young children diagnosed with spastic cerebral palsy between the ages 3-6 (Choe et al., 2018). Another study of the MBI assessed the functional ability of children with congenital muscular dystrophy and Barth syndrome between ages 5-19 (Lim et al., 2016). However, no studies to date have been conducted specifically evaluating the reliability and validity of the MBI for use with children who have congenital muscular dystrophy and Barth syndrome.
There are numerous studies investigating the psychometric properties of the MBI, a majority of these studies focus on adults with neurological conditions, such as patients that have experienced a stroke (Fricke & Unsworth, 1997; Ohura et al., 2017). In addition, the MBI has been found to be very effective for utilization with the adult population. However, there is still limited research to support whether it can also be beneficial for children with disabilities although significant findings from adult populations reveal that the MBI has the potential to be an effective and convenient tool to administer in pediatric settings (Choe et al., 2018; Ohura et al., 2017). Therefore, healthcare practitioners, such as occupational therapists, could potentially benefit from having an evidence-based assessment to quickly assess ADL performance in children with rare disorders.
The purpose of this study was to evaluate the psychometric properties of the MBI targeted children with rare disorders who experience muscle weakness as a common symptom. We hypothesized that the MBI is a valid and reliable assessment of functional mobility of children with rare disorders who have muscle weakness including Barth syndrome and congenital muscular dystrophy. Results from this study would contribute to pediatric healthcare practitioners who may use the MBI when evaluating ADL performance of children with rare disorders.
∐. Methods
1. Participants and procedure
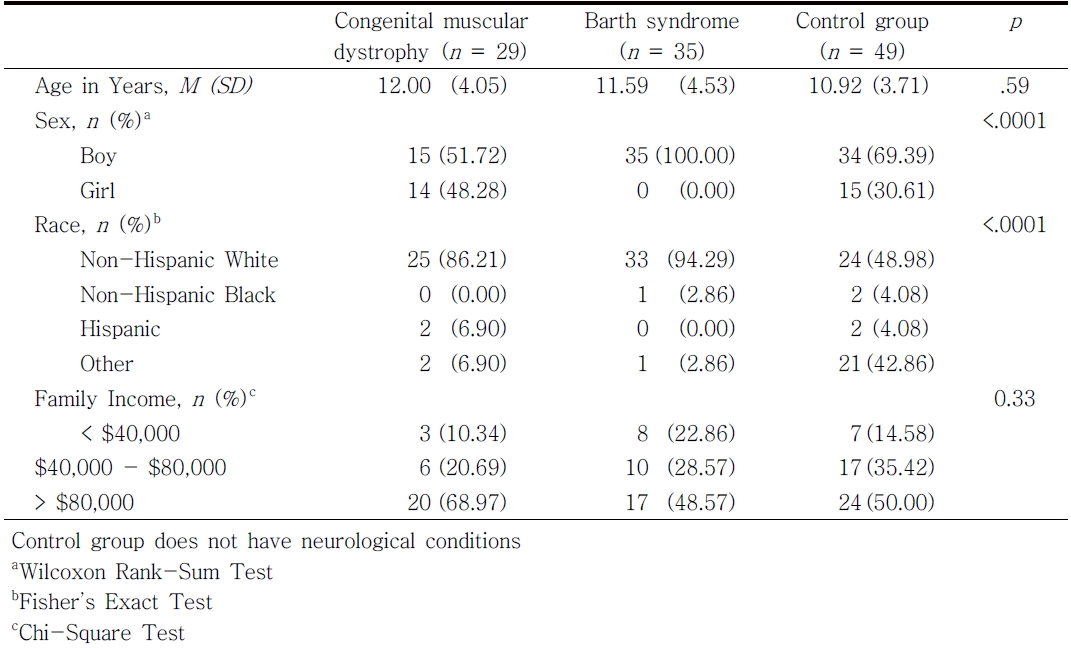
This study used a cross-sectional survey design. The target participants in this study were parents who have children or youth between the ages of 4 and 19 with congenital muscular dystrophy (n = 29; 12.00 years, SD = 4.05), Barth syndrome (n = 35; 11.59 years, SD = 4.53), and control group that consists of typically developing children without neurological disorders (n = 49; 10.92 years, SD = 3.71)(see Table 1). The recruitment and data collection were conducted from June 2014 to January 2015. Parents of children with Barth syndrome were recruited during their participation in the 7th International Scientific, Medical & Family Conference held by Barth Syndrome Foundation (BSF) on June 23-28, 2014 in Clearwater, FL. The protocol of this study was reviewed by the BSF research committee before the conference, and the data collection session was scheduled to be completed during the conference. The study letter and flyer together with a brief study introduction were sent to parents after approval from the BSF research committee. Parents of children with congenital muscular dystrophy were recruited by CureCMD and congenital muscular dystrophy studies at the University of Florida (UF) and the National Institutes of Health. Parents of unaffected children were recruited by word of mouth and by flyers posted on UF department websites and Listserv, Facebook groups, and HealthStreet (http://healthstreet.program.ufl.edu/). This study was originally approved by the University of Florida Institutional Review Board and reapproved by the Georgia State University Institutional Review Board. Written informed consent was obtained from parents.
Two types of questionnaires were used in this study: a paper-and-pencil version and an online version. The online version was administered by using Qualtrics, which is an online survey software. Parents completed either paper-and-pencil or online versions of the questionnaires at their convenience. Completion of the questionnaires took about 20 to 30 minutes. In this study, the parents of children with congenital muscular dystrophy and unaffected children completed the questionnaire on Qualtrics, and the parents of children with Barth syndrome completed a pencil-and-paper-version of the MBI due to limited access to the Internet at the conference.
2. Instrument
The Modified Barthel Index (MBI) is a 10-item questionnaire that measures the child’s performance in ADLs (i.e., child’s functional ability; Shah, Vanclay, & Cooper, 1989). The MBI consists of 10 scales, including 1) Personal Hygiene, 2) Bathing Self, 3) Feeding, 4) Toileting, 5) Stair Climbing, 6) Dressing, 7) Bowel Control, 8) Bladder Control, 9) Ambulation/Wheelchair, and 10) Chair/Bed Transfers. The MBI is administered by a therapist to a family member or friend who can respond to the items based on their observation of their child’s daily life. Each item is scored from unable to perform task to fully independent (0-5 for Personal Hygiene and Bathing Self; 0-10 for Feeding, Toilet Transfer, Dressing, Bladder Control, Bowel Control, and Stair Climbing; 0-15 for Chair/Bed Transfer and Ambulation/Wheelchair). The total score is obtained by the sum of 10 items, and ranges from 0 to 100. Higher scores indicate better functional ability or independence.
3. Data analysis
1) Descriptive statistics
Descriptive analyses were used to examine the central tendency (mean) and spread (standard deviations) of the MBI items. The ceiling and floor effect were examined based on a criterion of less than 15% of the sample scored at the extreme (McHorney & Tarlov, 1995). The difficulty index was calculated to examine an item difficulty hierarchy among the 10 MBI items (item mean divided by total item score; Anastasi & Urbina, 1997). The discriminative index was calculated using the item-to-total correlation based on Pearson’s product moment correlation coefficient. An acceptable discriminative index criterion was greater than .28 (Crocker & Algina, 1986; Portney & Watkins, 1993).
4. Psychometric properties
1) Reliability
Internal consistency reliability was determined for the MBI by calculating Cronbach’s α, where scales with reliabilities of .70 or greater are recommended as an acceptable level for comparing different groups. We considered a Cronbach’s α between .50 and .70 as moderate, and a Cronbach’s α less than 0.5 as small (Portney & Watkins, 1993).
2) Structural validity
Exploratory factor analysis was used to examine structural validity of the MBI. The Weighted Least Squares with adjustments for the Mean and Variance (WLSMV) estimation based on a polychoric correlations matrix was used to treat the categorical rating scale of the MBI (Revicki et al., 2014). Factor structure(s) was determined using eigenvalues, factor loadings (λ > 0.4), and model fit indices, including comparative fit index (CFI > 0.95 for good fit), Tucker–Lewis Index (TLI > 0.95 for good fit), root mean square error of approximation (RMSEA < 0.06 for good fit), and standardized root mean square residual (SRMR < 0.08 for good fit; Reeve et al., 2007). Mplus version 7.11 was used to perform the exploratory factor analysis (Muthén & Muthén, 2012).
3) Known group validity
The MBI score did not meet the normaility assuptiom. So, Kruskal–Wallis test was used as a non-parametric one-way ANOVA to examine the group differences in the total MBI score across three study groups. When there were significant differences among the three groups, we utilized Tukey’s honestly significant difference as a post-hoc test to identify differences in the pairwise comparisons. Cohen’s d was calculated to measure the effect size in the pairwise comparisons. We considered an effect size value of 0.20, 0.50 and greater than 0.80 as a small, moderate and large effect, respectively (Portney & Watkins, 1993).
Ⅲ. Results
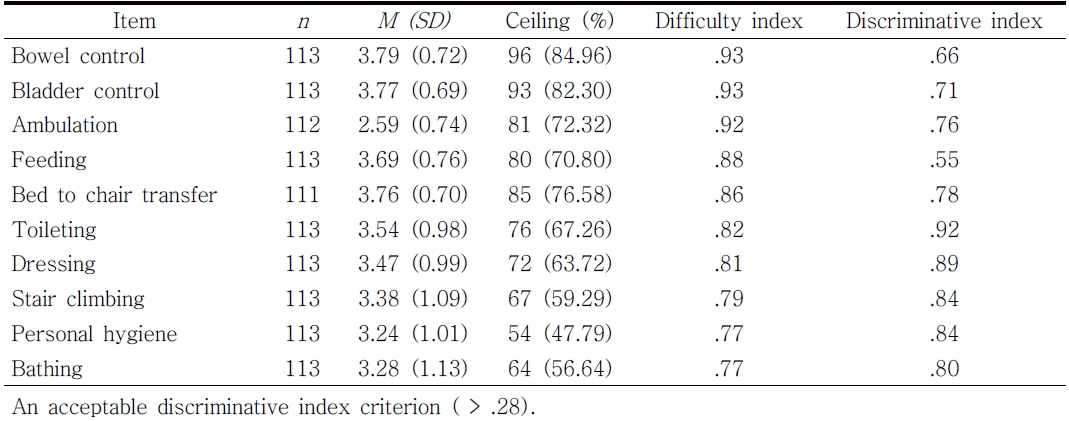
Table 2 presents descriptive statistics of the MBI items. All items demonstrated a high ceiling effect (range 56.6%-85.5%) indicating the study participants showed skewed response distributions toward the ceiling (fully independent). The difficulty index values among the 10 items ranged between .77 and .93. The most difficult items were bathing (.77) and personal hygiene (.77) whilst the easiest items were bowel control (.93) and bladder control (.93). The item discriminative indices of the MBI items ranged from .55 to .92. The most discriminative item was toileting (r = .92) whilst the least discriminative item was feeding (r = .55).
1. Internal consistency
The Cronbach’s α for the Barth syndrome group was .91, and the Cronbach’s α for the congenital muscular dystrophy group was .93; both levels demonstrate good internal consistency of the MBI for those with rare disorder diagnoses. In contrast, Cronbach's α for the control group was .67, indicating low levels of internal consistency for those in the control group.
2. Structural validity
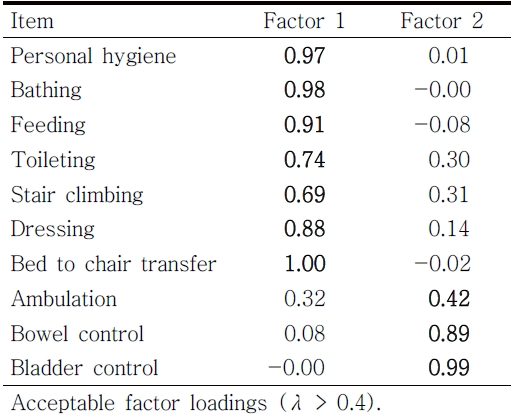
The exploratory factor analysis revealed that there were two-dominant factor structures among the MBI items. The two-factor structures model fit was excellent (RMSEA = 0.034, CFI = 1.00, TLI=1.00, SRMR = 0.02). The first and second eigenvalues were 8.46 and 0.68, respectively. Each item demonstrated acceptable factor loadings (λ > 0.4) on the dominant factor structure (see Table 3). The first factor was related to self-care functioning and the second factor were related to physiological needs/ambulation (bowel control, bladder control, and ambulation).
3. Known group validity
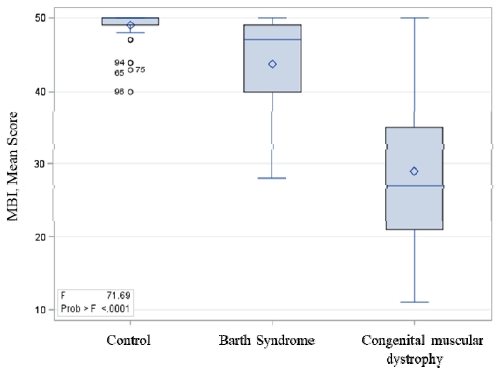
The Kruskal-Wallis Test revealed that there were significant differences across the three groups (F = 71.7, df = 2, p < .0001). The posthoc Tukey’s test revealed that the three groups were statistically different from each other (mean score: control = 49.0, Barth syndrome = 43.8, congenital muscular dystrophy = 28.9, p < .05) (see Figure 1). The effect size was large for the Barth syndrome and control group (Cohen’s d = 1.06) as well as the congenital muscular dystrophy and control group (Cohen’s d = 2.32).
Ⅳ. Discussion
The primary implication of our study includes whether the MBI is an effective measure to evaluate self-care and mobility ADLs in children with rare disorders. As other studies have revealed good to excellent reliability and validity of MBI in adult populations, there is a clinical need to determine whether this evidence-based and convenient assessment can be similarly effective when utilized with children (Fricke & Unsworth, 1997; Ohura et al., 2017). Therefore, our goal was to establish psychometric properties for the use of the MBI in children with rare disorders who experience muscle weakness as a common symptom.
Our findings suggest that reliable and valid inferences can be concluded from the MBI in terms of content for children with rare disorders. Internal consistency was excellent for the MBI in both the Barth syndrome population (Cronbach’s α = .91) and congenital muscular dystrophy population (Cronbach’s α = .91). Internal consistency was not high enough for the control group (Cronbach’s α = .67) but was close to cut-off score (Portney & Watkins, 1993). The known group validity demonstrated that the effect size was large for the Barth syndrome and control group (Cohen’s d = 1.06) as well as the congenital muscular dystrophy and control group (Cohen’s d = 2.32). In other words, this result revealed that the MBI has high construct validity and measures what it is intended to measure in these two populations with neurological muscle weakness. These results are similar to past studies of adults with localized neurological deficits from stroke or spinal cord injury (Anderson et al., 2008; Hobart & Thompson, 2001; Küçükdeveci et al., 2000; Ohura et al., 2017). Our new findings expand use of the MBI to children with diffuse neurological deficits from Barth syndrome and congentinital muscular dystrophy.
The MBI administered to children with rare disorders demonstrated two-dominant factor structures among the 10 items (self-care functioning and physiological needs/ambulation). While previous studies targeted different patient populations, the MBI demonstrated similar factor structures (Küçükdeveci et al., 2000; Leung, Chan, & Shah, 2007; Tennant, Geddes, & Chamberlain, 1996). Leung et al. (2007) examined the factor structures of the Chinese versions of the MBI with patients after stroke and reported that bowel control and bladder control present as a single factor. Two other studies based on Rasch analysis with stroke populations revealed that bowel control and bladder control misfit to the Rasch model indicting that these two items present another measurement construct (Küçükdeveci et al., 2000; Tennant et al., 1996). Interestingly, our study revealed that ambulation can be grouped with bowel control and bladder control. In addition, these three items demonstrated similar item difficulty and these items were relative challenging for the study sample. These study findings indicate that our study sample might need to perform ambulation to complete bowel and bladder control. Further support for this interpretation is provided by our finding of a high ceiling effect, which shows our participants were skewed toward independent. Therefore, it is likely bowel and bladder control included a more complex toileting routine that involved ambulation.
Generally, stair climbing is one of the most difficult daily tasks (Küçükdeveci et al., 2000; Leung et al., 2007; Tennant et al., 1996). Our study revealed that personal hygiene was a more challenging task compared to stair climbing. This finding indicates that children with rare disorders need more assistance to complete personal hygiene compared to stair climbing, which was the most challenging task for adult populations with chronic conditions. One interpretation of this difference is that participation in stair climbing may be more common in adults with chronic but stable neurologic conditions than children with neurodegenerative conditions (Jalali et al., 2008). Furthermore, our sample of children and their caregivers may value successful participation in different ADL than adults from previous studies. Typically, Rasch analysis can accurately estimate an item difficulty hierarchy among test items; however, we were not able to utilize those models because the MBI with current study sample did not meet the core assumption of unidimensionality for item response theory models and demonstrated multiple factor structures among the 10 MBI items. Although our results revealed that the MBI is a valid and reliable measure, there were still some limitations involving our study.
1. Limitations
One limitation was that our statistical analysis was conducted on a relatively small sample size for the standard factor analysis. In other words, the identified factor structures could be biased with an inflated type II error. A second limitation of our study was we did not divide our population samples further into subgroups, separating older and younger children which allow researchers to further test the stability of the instrument, such as differential item functioning across age groups. However, due to the small sample size, children of all ages were analyzed together in one group. A third limitation of the study was lack of variety of rare disorders among our sample. The study consisted of only children with a diagnosis of congenital muscular dystrophy or Barth syndrome. Due to these limitations, our findings should be interpreted with caution. A simulation study could partially address the issue of the small sample size (e.g., factor structure); however, this approach also raises a concern about unrealistically homogenous simulated data which could artificially inflate internal consistency results. Therefore, future research should include larger and more diverse sample sizes of children with various rare disorders other than congenital muscular dystrophy and Barth syndrome.
Ⅴ. Conclusion
A child’s performance in activities of daily living is an important indicator in assessing the progression of rare disorders. A psychometrically appropriate ADL scale that meets both research and clinical needs provides valuable information for demonstrating the evidence-based value of occupational therapy interventions. Our study was the first to evaluate the psychometric properties of the MBI when applied to children with rare disorders. Based on the study findings, health policymakers could use the study results for revising the current health policies and improving these children’s quality of life. Overall, the results of this study indicate that the MBI is reliable and valid when used with children with rare disorders.